Simple Serial Dilution Calculation
Resource Materials: Making Simple Solutions and Dilutions 1. Simple Dilution (Dilution Factor Method based on ratios) A simple dilution is one in which a unit volume of a liquid material of interest is combined with an appropriate volume of a solvent liquid to achieve the desired concentration. The dilution factor is the total number of unit volumes in which your material will be dissolved. The diluted material must then be thoroughly mixed to achieve the true dilution.
Simple Serial Dilution Calculation Ppt
For example, a 1:5 dilution (verbalize as '1 to 5' dilution) entails combining 1 unit volume of solute (the material to be diluted) + 4 unit volumes of the solvent medium (hence, 1 + 4 = 5 = dilution factor). The dilution factor is frequently expressed using exponents: 1:5 would be 5e-1; 1:100 would be 10e-2, and so on. Example 1: Frozen orange juice concentrate is usually diluted with 4 additional cans of cold water (the dilution solvent) giving a dilution factor of 5, i.e., the orange concentrate represents one unit volume to which you have added 4 more cans (same unit volumes) of water. So the orange concentrate is now distributed through 5 unit volumes.
This would be called a 1:5 dilution, and the OJ is now 1/5 as concentrated as it was originally. So, in a simple dilution, add one less unit volume of solvent than the desired dilution factor value. Example 2: Suppose you must prepare 400 ml of a disinfectant that requires 1:8 dilution from a concentrated stock solution with water. Divide the volume needed by the dilution factor (400 ml / 8 = 50 ml) to determine the unit volume. The dilution is then done as 50 ml concentrated disinfectant + 350 ml water. Serial Dilution A serial dilution is simply a series of simple dilutions which amplifies the dilution factor quickly beginning with a small initial quantity of material (i.e., bacterial culture, a chemical, orange juice, etc.). The source of dilution material (solute) for each step comes from the diluted material of the previous dilution step.
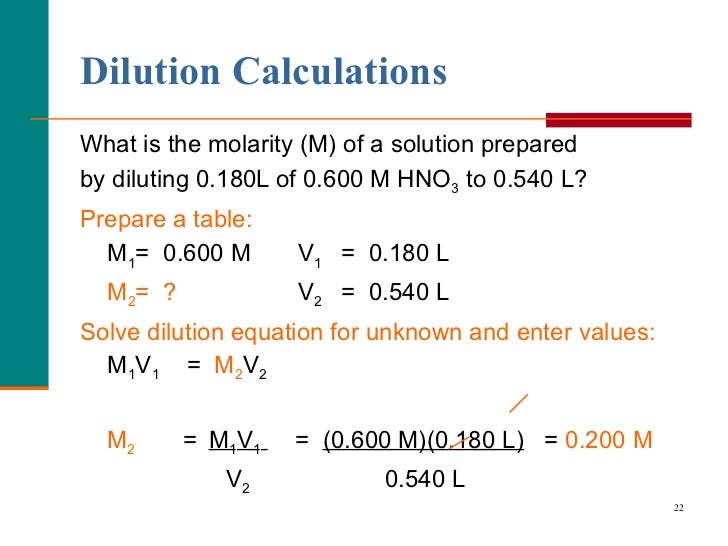
In a serial dilution the total dilution factor at any point is the product of the individual dilution factors in each step leading up to it. Final dilution factor (DF) = DF 1.
DF 2. DF 3 etc. Example: In a typical microbiology exercise the students perform a three step 1:100 serial dilution of a bacterial culture (see figure below) in the process of quantifying the number of viable bacteria in a culture (see figure below). Each step in this example uses a 1 ml total volume. The initial step combines 1 unit volume of bacterial culture (10 ul) with 99 unit volumes of broth (990 ul) = 1:100 dilution.

In the second step, one unit volume of the 1:100 dilution is combined with 99 unit volumes of broth now yielding a total dilution of 1:100x100 = 1:10,000 dilution. Repeated again (the third step) the total dilution would be 1:100x10,000 = 1:1,000,000 total dilution. The concentration of bacteria is now one million times less than in the original sample. Making fixed volumes of specific concentrations from liquid reagents: V 1 C 1 =V 2 C 2 Method Very often you will need to make a specific volume of known concentration from stock solutions, or perhaps due to limited availability of liquid materials (some chemicals are very expensive and are only sold and used in small quantities, e.g., micrograms), or to limit the amount of chemical waste.
The formula below is a quick approach to calculating such dilutions where: V = volume, C = concentration; in whatever units you are working. (stock solution attributes) V 1 C 1 =V 2 C 2 (new solution attributes) Example: Suppose you have 3 ml of a stock solution of 100 mg/ml ampicillin (= C 1 ) and you want to make 200 ul (= V 2 ) of solution having 25 mg/ ml (= C 2 ). You need to know what volume ( V 1 ) of the stock to use as part of the 200 ul total volume needed. V 1 = the volume of stock you will start with.
This is your unknown. C 1 = 100 mg/ ml in the stock solution V 2 = total volume needed at the new concentration = 200 ul = 0.2 ml C 2 = the new concentration = 25 mg/ ml By algebraic rearrangement: V 1 = ( V 2 x C 2 ) / C 1 V 1 = (0.2 ml x 25 mg/ml) / 100 mg/ml and after cancelling the units, V 1 = 0.05 ml, or 50 ul So, you would take 0.05 ml = 50 ul of stock solution and dilute it with 150 ul of solvent to get the 200 ul of 25 mg/ ml solution needed. Remember that the amount of solvent used is based upon the final volume needed, so you have to subtract the starting volume form the final to calculate it. Moles and Molar solutions (unit = M = moles/L) Sometimes it may be more efficient to use molarity when calculating concentrations. A mole is defined as one gram molecular weight of an element or compound, and comprised of exactly 6.023 x 10^23 atoms or molecules (this is called Avagadro's number). The mole is therefore a unit expressing the amount of a chemical. The mass (g) of one mole of an element is called its molecular weight (MW).
When working with compounds, the mass of one mole of the compound is called the formula weight (FW). The distinction between MW and FW is not always simple, however, and the terms are routinely used interchangeably in practice. Formula (or molecular) weight is always given as part of the information on the label of a chemical bottle. The number of moles in an arbitrary mass of a dry reagent can be calculated as: # of moles = weight (g)/ molecular weight (g) Molarity is the unit used to describe the number of moles of a chemical or compounds in one liter (L) of solution and is thus a unit of concentration.
By this definition, a 1.0 Molar (1.0 M) solution is equivalent to one formula weight (FW = g/mole) of a compound dissolved in 1 liter (1.0 L) of solvent (usually water). Example 1: To prepare a liter of a simple molar solution from a dry reagent Multiply the formula weight (or MW) by the desired molarity to determine how many grams of reagent to use: Chemical FW = 194.3 g/mole; to make 0.15 M solution use 194.3 g/mole. 0.15 moles/L = 29.145 g/L Example 2: To prepare a specific volume of a specific molar solution from a dry reagent A chemical has a FW of 180 g/mole and you need 25 ml (0.025 L) of 0.15 M (M = moles/L) solution. How many grams of the chemical must be dissolved in 25 ml water to make this solution? # grams /desired volume (L) = desired molarity (mole/L). FW (g/mole) by algrebraic rearrangement, # grams = desired volume (L). desired molarity (mole/L).
FW (g/mole) #grams = 0.025 L. 0.15 mole/L. 180 g/mole after cancelling the units, #grams = 0.675 g So, you need 0.675 g /25 ml For more on molarity, plus molality and normality: More examples of worked problems: 5. Percent Solutions (% = parts per hundred or grams/100 ml) Many reagents are mixed as percent concentrations as weight per volume for dry reagent OR volume per volume for solutions. When working with a dry reagent it is mixed as dry mass (g) per volume and can be simply calculated as the% concentration ( expressed as a proportion or ratio) x volume needed = mass of reagent to use.
Example 1: If you want to make 200 ml of 3% NaCl you would dissolve 0.03 g/ml x 200 ml = 6.0 g NaCl in 200 ml water. When using liquid reagents the percent concentration is based upon volume per volume, and is similarly calculated as% concentration x volume needed = volume of reagent to use. Example 2: If you want to make 2 L of 70% actone you would mix 0.70 ml/ml x 2000 ml = 1400 ml acetone with 600 ml water. To convert from% solution to molarity, multiply the% solution by 10 to express the percent solution grams/L, then divide by the formula weight. Molarity = (grams reagent/100 ml). 10 xxxxxxxxxx FW Example 1: Convert a 6.5% solution of a chemical with FW = 325.6 to molarity, (6.5 g/100 ml). 10 / 325.6 g/mole = 65 g/L / 325.6g/mole = 0.1996 M To convert from molarity to percent solution, multiply the molarity by the FW and divide by 10:% solution = molarity.
Huawei e3531 driver. FW xxxxxxxxxx 10 Example 2: Convert a 0.0045 M solution of a chemical having FW 178.7 to percent solution: 0.0045 moles/L. 178.7 g/mole / 10 = 0.08% solution 6. Concentrated stock solutions - using 'X' units Stock solutions of stable compounds are routinely maintained in labs as more concentrated solutions that can be diluted to working strength when used in typical applications. The usual working concentration is denoted as 1x. A solution 20 times more concentrated would be denoted as 20x and would require a 1:20 dilution to restore the typical working concentration. Example: A 1x solution of a compound has a molar concentration of 0.05 M for its typical use in a lab procedure.
A 20x stock would be prepared at a concentration of 20.0.05 M = 1.0 M. A 30X stock would be 30.0.05 M = 1.5 M. Normality (N): Conversion to Molarity Normality = n.M where n = number of protons (H+) in a molecule of the acid.
Example: In the formula for concentrated sulfuric (36 N H2SO4), there are two protons, so, its molarity= N/2. So, 36N H2SO4 = 36/2 = 18 M. Modified 9-27-12 ga, Lewiston, ME 04240.
Huge Micro Problem As you know, bacteria are everywhere, invisible to the naked eye, yet influencing every environment on Earth. What happens when you need to know how many individual bacterial cells are contaminating a food, living in an environmental sample, or growing in a culture tube?
You need some method for counting the bacteria accurately. But, it is not uncommon for a liquid culture of bacteria to have a billion cells in every milliliter of media. Think about that for one second. In your kitchen, you probably have a teaspoon. Every teaspoon has about 5 milliliters.
That means that every teaspoon of liquid could potentially have 5 billion bacteria in it. Even if you counted one bacteria every second, it would take you over 150 years to get to 5 billion! Obviously, this is not a viable option. So, what can you do? You need fewer bacteria to count.
Ideally, you want to only have to count between 30 and 300 bacteria, a range of numbers that takes only at most a few minutes to count. But, how do we get there? Serial Dilution The answer is through dilution. If you simply pull out a smaller, exact quantity of culture liquid, you could count those bacteria and, based on how much you pulled out of the total, you can determine how many bacteria are in your original sample. Sounds easy, right? But first, one more analogy: you have billions of bacterial cells and need to get down to 30 to 300.

In order to do that, you would have to dilute your sample about 10 million-fold. To do this, you would need to take about 15 milliliters of your sample, about 3 teaspoons, and dilute it into your swimming pool! I doubt this is a viable option, especially if you're working in a cramped lab space. So instead, let's not dilute just once. We can dilute once, then dilute this dilution, only to dilute this dilution, and so on until we get to the appropriate concentration of cells. This is called a serial dilution. A serial dilution is a series of sequential dilutions used to reduce a dense culture of cells to a more usable concentration.
Each dilution will reduce the concentration of bacteria by a specific amount. So, by calculating the total dilution over the entire series, it is possible to know how many bacteria you started with. The best way to fully grasp serial dilutions is to try out the procedure yourself. How to Perform a Serial Dilution I'm going to walk you through an example serial dilution using the easiest method, but, once you grasp the concept, you can change the actual numbers to whatever works best for you and do it the same way.
To start, we need 10 milliliters (10 ml) of your original bacterial culture (labeled OBC). Before we start diluting, we need to prepare several dilution blanks, which are tubes containing your diluting liquid in exact quantities.
Your liquid could be growth media, saline, sterile water, or any other appropriate liquid. For this example, we need 5 dilution blanks, numbered 1-5. In each tube, we need exactly 9 ml of liquid media. The reason we need 9 ml will become apparent soon. The tubes should be lined up like this: How to line up tubes for serial dilution example The first step is to gently shake or swirl the tube. This will ensure that your cells are evenly distributed in the tube.
If your cells settle to the bottom, and you remove liquid without swirling, you run the risk of not getting enough cells, invalidating your final count. Remember to always swirl the tube before removing liquid. Once swirled, carefully transfer exactly 1 ml from your OBC Tube to Tube 1. Now, you should have 10 ml of liquid in Tube 1. Exactly one-tenth of your cells are now in a new tube with a final volume of 10 ml.
You just performed a 1 in 10 dilution, or it could be written 1/10. 1 is the volume you transferred, and 10 is the final volume of the tube after the transfer. Now, you are done with tube OBC, and Tube 1 becomes the next tube to be diluted. Like we did before, swirl your tube before transferring 1 ml from Tube 1 into Tube 2. Again, exactly one-tenth of your cells in Tube 1 are transferred to Tube 2, with a final volume of 10 ml. Tube 1 should have exactly 9 ml left. Tube 2 now contains a 1 in 10 dilution of Tube 1.
In order to calculate the total dilution from Tube OBC, simply multiply your two dilutions: 1/10 X 1/10 = 1/100. So far, you have performed a 1/100 dilution from the original bacterial culture. You want to follow the same procedure for the remaining dilution blanks: 1 ml from Tube 2 is transferred to Tube 3; 1 ml from Tube 3 is transferred to Tube 4; and, finally, 1 ml from Tube 4 is transferred to Tube 5. Each transfer is another 1 in 10 dilution. To calculate the final dilution, simply multiply all the dilutions together: 1/10 X 1/10 X 1/10 X 1/10 X 1/10 = 1/100,000. Now we might have a reasonable number of cells to count. To finish the technique, let's imagine we count the cells in Tube 5 and find 50 total cells, right in the desired 30 to 300 zone.
In order to determine how many cells we started with in our original culture, all you need to do is multiply the cell count by the total dilution: 50 X 100,000 = 5,000,000 bacterial cells in our original 10 ml sample. I bet you would rather count to 50 than 5 million! It is important to note that you can use any volumes here. If your dilution blanks are 6 ml, and you are transferring 1 ml, the dilution would be 1/7. The math is exactly the same: 1/7 X 1/7 X 1/7 X 1/7 = 1/2401. You also don't need to have the same final volume in every tube.
You can dilute 1/2, then 1/5, then 1/8. Simply multiply as before to get the final dilution: 1/2 X 1/5 X 1/8 = 1/80. Now you might be wondering how we count the tiny cells. There are several methods that could be used, but we will use the culture plate method. We can take a sample of Tube 5 and grow the cells on a culture plate. Then, once the bacteria start to grow, they form colonies that eventually get large enough to see.
Then, we can count the colonies and back calculate to find the original concentration of bacteria in our sample. You have solved what started out to be a pretty daunting problem! Lesson Summary Let's briefly review serial dilutions.
A serial dilution is a series of sequential dilutions used to reduce a dense culture of cells to a more usable concentration. The easiest method is to make a series of 1 in 10 dilutions. In this method, exactly 1 ml of each successive dilution is transferred into exactly 9 ml of liquid in a dilution blank, creating a 1/10 dilution. In order to calculate the final dilution, all you need to do is multiply the 1/10 X 1/10, continuing like this for each step in the dilution. To determine exactly how many cells you started with, simply take the number of colonies you counted, and multiply it by your final dilution factor. Learning Outcomes Once you've completed this lesson, you may be able to:. Create a serial dilution and recognize its purpose.
Identify the steps necessary for developing a serial dilution. Know how to count bacteria cells.